So far in this series we have looked at where your can spear and what you can spear. Next up we’re going to take a look at what gear you’re going to need to go spearfishing.
What gear should I buy?
Be prepared to spend a bit of money on your gear. Cheap gear is cheap for a reason. Talk to some locals or get onto some forums and see what other people are using and saying about their equipment. If possible, try to borrow some gear before you invest in your own kit. Most experienced spearo’s will have spare gear laying around. Talk to them and see what they liked/didn’t like about that gear. You can build up to better gear to some extent, but there are some basics that you will need to get started.
Mask and snorkel
If you’re considering getting into spearfishing, you’ve probably done some snorkelling or diving. It’s fine to use a mask and snorkel that you’ve bought for this purpose however there are a few things you may want to consider for a spearfishing mask when it comes to upgrading or buying your first one. Firstly, a black (or any non-clear colour) silicone skirt. This will stop reflections on your mask lens and enable you to spot fish better from the surface. It sounds like a minor annoyance, but it’s one of those little things that can make a big difference. Second, you want a low volume mask. A low volume mask requires less air to equalise at depth and will sit closer to your eyes giving you a better field of vision. lastly and by far the least important are mirrored or shaded lenses. These are totally non-essential, but fish will tend to come in closer when they can’t see you looking at them. I have always owned masks with clear lenses but it means that I track fish in my peripheral vision and only look directly at them when I’m ready to shoot.

Comparison of freediving (left) and scuba diving (right) masks
Snorkels – In my experience, it doesn’t really matter. Try to avoid those big splash guards on the top as they tend to make the snorkel flop around. Learn to use your tongue as a splash guard instead. A stiff snorkel is better for spearing as it won’t flop around as much. Also, don’t rely on those plastic clips to attach the snorkel to your mask. Get one of the old-school silicone ones or simply slip the snorkel under your mask strap.
Fins

Comparison of diving (top) and freediving (bottom) fins. Note: the split in the diving fin is not appropriate for spearfishing.
You can use pretty much anything to get you started but you will probably want to upgrade as soon as you can. Good fins will make a big difference in your breath holding ability. That being said, I know people who use body board fins for spearfishing. Proper freediving fins are much more energy efficient which is why a good pair will help a lot with your breath hold and you’ll be able to chase faster fish with them too. The down side is that a decent set are going to cost you. split-fins, often used for scuba diving are not so great for spearfishing. They are ok in terms of speed and energy, however your float line can get very easily tangled between the blades. For that reason, I really would not recommend this style of fin. Full foot or strap? I prefer full foot for spearfishing as it means less neoprene around my feet – you use a thin sock rather than wetsuit booties. However some rock hoppers might prefer something more solid on their feet for getting to their dive sites.
Wetsuit
 Again, pretty much any wetsuit will be fine to get you started as long as it’s an appropriate thickness for the water temperature where you dive. When you decide it’s time to invest in a good wetsuit, there are a couple of things to consider. Firstly, 1-piece or 2-piece? Most spearfishing wetsuits are 2-piece. I don’t really know why. I did use my 1-piece scuba diving wetsuit for spearfishing for years before upgrading so I don’t think there is any real issue there. Just good to know that both types exist. Secondly, open or closed cell neoprene? Most spearo’s I know like the open cell wetties because they’re warmer and allow for a greater range of movement. However, they are harder to get into (you’ll need to lube yourself up) and they are less durable than their closed cell counterparts. Next, think about the colour. I used to laugh at people in camo wetsuits until I saw one in action. I watched my buddy almost disappear into a bed of kelp. Now I wear a camo wetsuit. Whether you like camouflage or not, darker colours are better, but at the end of the day, get something that is comfortable for you and suitable for the area you dive.
Again, pretty much any wetsuit will be fine to get you started as long as it’s an appropriate thickness for the water temperature where you dive. When you decide it’s time to invest in a good wetsuit, there are a couple of things to consider. Firstly, 1-piece or 2-piece? Most spearfishing wetsuits are 2-piece. I don’t really know why. I did use my 1-piece scuba diving wetsuit for spearfishing for years before upgrading so I don’t think there is any real issue there. Just good to know that both types exist. Secondly, open or closed cell neoprene? Most spearo’s I know like the open cell wetties because they’re warmer and allow for a greater range of movement. However, they are harder to get into (you’ll need to lube yourself up) and they are less durable than their closed cell counterparts. Next, think about the colour. I used to laugh at people in camo wetsuits until I saw one in action. I watched my buddy almost disappear into a bed of kelp. Now I wear a camo wetsuit. Whether you like camouflage or not, darker colours are better, but at the end of the day, get something that is comfortable for you and suitable for the area you dive.
Gloves
Quite possibly the most contentious issue in spearfishing! At the end of the day, gloves aren’t going to cost you an arm and a leg so try a few and find a pair that work for you. But you will need a pair of gloves. Some fish are very spiky so gloves will help to prevent a painful encounter. Also you’ll most likely be around rocky reefs or some kind of hard substrate so gloves will mitigate scrapes and cuts and make entries and exits from a rocky shore much less painful.
Weightbelt
Anything will get the job done here, but most spearo’s prefer the rubber type as they don’t slide around as much. Some people like a crotch strap to keep it in place. Other people love weight vests as you can distribute the weights evenly around your body. A downside to vests is that they may not ditch as easily in an emergency. Be sure to position your weights toward the back so that if you pass out, your weight belt will roll you onto your back. Never overweight yourself! If you’re negatively buoyant at the surface, you’re wearing too much weight. You should be able to float with your collarbone above water when you position yourself vertically and take a breath in. It’s worth taking that extra bit of time to get your weights set up correctly before you head out into deeper water.
Spear or Speargun
Hand spears
Some people love them. Most people start out with them. Some people I know tow one behind their float for flathead or fish inside caves. Honsetly, I can’t tell you much about these as I haven’t used one for a really long time. Basically you can buy them with aluminium or fibreglass shafts. They are cheaper than a speargun and you will catch some fish using them. However, they have less range and in my limited experience tend to be less accurate, though that probably comes down to the user.
Spearguns
Spend some money here and get something decent. Do some research and find out the pro’s and con’s of each brand and each model. A cheap gun will be less accurate and won’t last you very long. They are also quite inconsistent in their aim which makes it very difficult for a beginner to develop that skill – what worked last time may not work next time. Look for a gun with rails to guide the spear.
Size matters, but bigger is not always better. Long guns will give you longer range (generally) and tend to have more ‘punch’. Shorter guns are easier to track side to side. Have a think about what kind of fish you want to target. Long guns are generally better for bigger pelagics whereas shorter guns tend to be better for reef fish darting in and out of the rocks. Shorter guns are generally easier to load. Don’t agonize too much over length though. You’ll probably go through a few until you find a size that suits your style. 100 to 110 cm guns are probably a good starting point. I have used a 120 cm gun around rocky reefs to great effect and I’ve brought in some good sized kingfish on a little 90 cm.

Single or double rubbers? It comes down to what you’re targeting. I find a single rubber suits me for most of my fishing, however my gun can accommodate a second. I like this configuration because it allows me to attach a second rubber to my gun as a spare in case one of them breaks. Having both rubbers loaded won’t give you a longer range, but it will give the spear more ‘punch’ and the spear will reach your target quicker so the second rubber can be good for targeting faster moving species.
Reels or wrapped line? Never used a reel so I can’t really comment. A reel won’t increase your range. The spear will lose momentum well before it reaches the end of a wrapped shooting line. However if you’re wanting to shoot bigger fish, a reel will probably help you land it. Comments on reels welcome! Bungee or no bungee? Doesn’t really matter. A bungee is a small piece of rubber attached between the shooting line and the muzzle. I like them because it makes reloading the shooting line easier. More importantly it dampens the movement of a struggling fish meaning less tear-offs while you’re landing a fish.

Open or closed muzzle? Open muzzles allow you to aim down the full length of the spear and make reloading the spear a bit quicker as you don’t need to thread the spear through the hole in the muzzle. The line wrap is a little more complicated though as the shooting line is used to hold the spear to the barrel. The shooting line which holds down the spear on an open muzzle may come loose in rough conditions and you need to be spot on with the length of the shooting line when you replace it. You can’t use a bungee on an open muzzled gun. Closed muzzles may take a little longer to reload, but I’ve never really considered this to be a big problem on my closed muzzle guns. While you can’t see down the full length of the spear for aiming with a closed muzzle, you do get used to it and I certainly haven’t had any issues with accuracy using a closed muzzle.
Roller guns are becoming popular and are worth a mention. I have never used one so would love to hear from anyone who has. The idea of these guns is that the length of the barrel can be reduced while still facilitating a longer rubber, giving more power with the advantages of a shorter gun. The spear also gets a straighter pull from the rubbers and hence greater accuracy. They seem a little more complicated to reload and maintain, but certainly nothing that couldn’t be learned from a YouTube tutorial.
A note on safety
Never ever point your spear or speargun at another person. In fact, don’t even point it at a fish unless you’re going to shoot it. Treat it like a firearm. Even though most spearguns come with a safety catch, there is no guarantee that it will hold. These things are not toys. They are killing implements. Treat them with respect.
Float and line
Yes you need these! Anyone who’s ever driven a boat knows how difficult it can be to spot a person in the water. For this reason your float should be a bright colour and stand out (unlike your wetsuit). Get a float that comes with a flag, preferably on a giant pole sticking way above the water. You may need to add a ballast weight to the bottom of your float to stop it rolling flag-side down. Streamlined floats are easier to tow, however I started out with a couple of spray painted milk bottles. Not ideal, but they got me started.
My float is probably my favourite bit of gear. It’s a great place to store a bunch of things. I keep an abalone knife and a mesh bag on mine as well as a photocopy of my fishing licence in a waterproof bag. One of the requirements in NSW is that you must carry your licence with you when you’re fishing. Obviously you don’t want to take your wallet with you into the water, so a lot of people just write their licence number on their float. This should satisfy most reasonable fisheries officers. Make sure you have your actual fishing licence in your car though.
You can use pretty much any kind of synthetic rope for your float line. It should be appropriate for the depth you want to dive to. Too much line will get tangled and your float will probably be up on the shore. Too little line and you won’t be able to get the bottom. You need buoyant rope. If your line sinks, you’ll spend all of your time trying to untangle it from rocks and weed. Your line also needs to be thin enough to thread your fish onto but not so thin that it breaks off in the swell. Five to eight millimeters is a good diameter. One end of the line should be attached to your gun and the other end to your float. Most people I know string their fish on their float line. You can get a neat bit of gear called a speed spike which allows you to thread your fish (through the gill slit and out the mouth) onto your line more easily. I highly recommend getting one of these.
Knife

Spearfishing knives (left and middle) vs diving knife (right). The Mac Sub 11D is on the left.
You need a knife for spearfishing. Firstly to kill your fish (if you don’t kill it outright) and secondly in case of entaglement. Remember, you will be towing a float and there is the potential for an entanglement. For that reason your knife needs to be in an accessible location on your body. I don’t like knives on the leg. I know you think it looks cool with a big dagger strapped to your calf, but it is more difficult to get to. A lot of spearo’s strap their knife to their forearm or upper arm. Personally I like mine on my belt. Get a knife with a slender blade and a fine point. This is what you will use to kill the fish. Typical scuba diving knives have a broader blade and these can work for spearfishing but they’re not great. The Mac Sub 11D is a great starting point that won’t break the budget.
Summary
Well, I think that just about covers all of the gear that you will need to get you started. I may do another post on ‘nice-to-have’ gear later on. I know that it’s very tempting to look for the cheapest gear you can find when starting out and that is ok in some areas. but I think you can save yourself some money in the long run if you invest in decent gear. The speargun and fins are probably the two areas that it’s really worth spending the extra cash.


 Blue wrasse aka blue groper are off limits to spearo’s. This is because they are a naturally inquisitive fish and will swim right up to a diver to check them out. For this reason, a lot of newbies get caught out and get excited about the great big fish right at the end of their spear. DO NOT shoot these fish. Be aware that the female blue gropers (they change sex to male when the dominant male in the area dies) are brown or yellow in colour. Learn to identify this species and do not shoot them. You can have a lot of fun when these fish are around. They will hang around and check you out. Try tapping your knife on a rock – they will often swim in very close to you to see what you’re doing. Some people like to cut up sea urchins for the gropers but I advise against this practice. These fish are very capable of feeding themselves and cutting up urchins is an unnecessary waste of life.
Blue wrasse aka blue groper are off limits to spearo’s. This is because they are a naturally inquisitive fish and will swim right up to a diver to check them out. For this reason, a lot of newbies get caught out and get excited about the great big fish right at the end of their spear. DO NOT shoot these fish. Be aware that the female blue gropers (they change sex to male when the dominant male in the area dies) are brown or yellow in colour. Learn to identify this species and do not shoot them. You can have a lot of fun when these fish are around. They will hang around and check you out. Try tapping your knife on a rock – they will often swim in very close to you to see what you’re doing. Some people like to cut up sea urchins for the gropers but I advise against this practice. These fish are very capable of feeding themselves and cutting up urchins is an unnecessary waste of life.










 There are lots of examples in nature of animals that form social groups. These species gain advantages and incur disadvantages from their social behaviour. For example an advantage might be better predator detection while foraging (known as the “many eyes” hypothesis) while a disadvantage could include higher rates of disease transmission. Studies suggest that the evolution and maintenance of sociality is likely to be influenced by environmental factors. Changes in the environment, like those caused by extreme weather events, are therefore likely to impact upon the social organisation of social species.
There are lots of examples in nature of animals that form social groups. These species gain advantages and incur disadvantages from their social behaviour. For example an advantage might be better predator detection while foraging (known as the “many eyes” hypothesis) while a disadvantage could include higher rates of disease transmission. Studies suggest that the evolution and maintenance of sociality is likely to be influenced by environmental factors. Changes in the environment, like those caused by extreme weather events, are therefore likely to impact upon the social organisation of social species. My research focuses on the coral gobies at Lizard Island, Queensland. Coral gobies are small fish, approximately three to four centimeters in length and they spend their entire adult lives within the branches Acroporid corals (corals of the genus Acropora). They suffer high mortality outside of their corals, and as such rarely move between corals once they have established themselves. I have observed up to 16 species of coral goby at Lizard Island which range in social organisation from strictly pair-forming species (which I will refer to as ‘Asocial’ species) to highly social species which can be found in groups of 12 or more (the largest group I’ve found was over 20 individuals).
My research focuses on the coral gobies at Lizard Island, Queensland. Coral gobies are small fish, approximately three to four centimeters in length and they spend their entire adult lives within the branches Acroporid corals (corals of the genus Acropora). They suffer high mortality outside of their corals, and as such rarely move between corals once they have established themselves. I have observed up to 16 species of coral goby at Lizard Island which range in social organisation from strictly pair-forming species (which I will refer to as ‘Asocial’ species) to highly social species which can be found in groups of 12 or more (the largest group I’ve found was over 20 individuals). During my studies, two cyclones have impacted my sites at Lizard Island which has been quite disruptive to my research, but has also presented a rare opportunity to gain an insight into the rarely studied effects of cyclones on social organisation. There is no doubt (unless you’re a cyclone skeptic) that cyclones cause severe damage to the physical structure of the reef. This destruction obviously has impacts on the abundance, diversity and distribution of reef species following the event. For example, obligate reef-dwelling species (species which depend on the structure of coral reefs for protection and food) tend to decrease in abundance while algal grazers tend to increase in abundance. However we know relatively little about how these events affect social structures of reef inhabitants which is a potential driver of the diversity and abundance patterns we observe. As I previously mentioned, social organisation is important in determining factors such as reproduction, foraging success and survival, all of which are critical for a species’ recovery from a major disturbance.
During my studies, two cyclones have impacted my sites at Lizard Island which has been quite disruptive to my research, but has also presented a rare opportunity to gain an insight into the rarely studied effects of cyclones on social organisation. There is no doubt (unless you’re a cyclone skeptic) that cyclones cause severe damage to the physical structure of the reef. This destruction obviously has impacts on the abundance, diversity and distribution of reef species following the event. For example, obligate reef-dwelling species (species which depend on the structure of coral reefs for protection and food) tend to decrease in abundance while algal grazers tend to increase in abundance. However we know relatively little about how these events affect social structures of reef inhabitants which is a potential driver of the diversity and abundance patterns we observe. As I previously mentioned, social organisation is important in determining factors such as reproduction, foraging success and survival, all of which are critical for a species’ recovery from a major disturbance.
 We found that social species decreased in group size following each cyclone while asocial species group size remained the same. This indicates that group size decreases observed in social species were unlikely due to direct mortality from the cyclones (otherwise we would have seen a corresponding drop in average group size in the asocial species as well). A year after the first cyclone, the social species had returned to their pre-cyclone group sizes (keep this point in mind as I’ll return to this in a minute). However, a year after the second cyclone the social species had not returned to pre-cyclone group sizes. This may indicate that multiple impacts have longer lasting effects on social organisation.
We found that social species decreased in group size following each cyclone while asocial species group size remained the same. This indicates that group size decreases observed in social species were unlikely due to direct mortality from the cyclones (otherwise we would have seen a corresponding drop in average group size in the asocial species as well). A year after the first cyclone, the social species had returned to their pre-cyclone group sizes (keep this point in mind as I’ll return to this in a minute). However, a year after the second cyclone the social species had not returned to pre-cyclone group sizes. This may indicate that multiple impacts have longer lasting effects on social organisation.
 This hypothesis looks at ecological factors which might constrain dispersal from a territory such as a lack of available habitat or high predation pressure. In relation to my work, one of the reasons that the social gobies might have re-formed their large social groups in smaller corals could be that they were constrained by a lack of available habitat. i.e. Gobies displaced by the cyclone might have had no choice, but to move into a coral which already had a small group of fish living in it. In this case, we would expect to see that most of the corals would be inhabited because vacant corals would be quickly taken up by gobies dispersing from crowded corals.
This hypothesis looks at ecological factors which might constrain dispersal from a territory such as a lack of available habitat or high predation pressure. In relation to my work, one of the reasons that the social gobies might have re-formed their large social groups in smaller corals could be that they were constrained by a lack of available habitat. i.e. Gobies displaced by the cyclone might have had no choice, but to move into a coral which already had a small group of fish living in it. In this case, we would expect to see that most of the corals would be inhabited because vacant corals would be quickly taken up by gobies dispersing from crowded corals.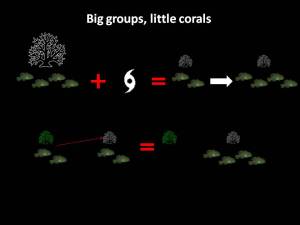 This hypothesis looks at the idea that animals gain some benefit of remaining on a site that outweighs the benefits of dispersing. For example, the site might be of a high quality which improves the animal’s fitness to survive and reproduce. Dispersing from this site risks, losing this benefit, unless it can find a site which confers the same or better benefits. In my project, it is likely that there was a lot of variation in coral quality following the cyclone. While fish might have quickly moved into whatever shelter they could find, they might have realised later on that their coral was not very good (indicated by the green, algae covered coral in the diagram), and decided that it was more beneficial to vacate their low quality coral and move into a high quality coral (white coral in the diagram) with an existing group of fish. In this scenario, we would expect to find a lower proportion of inhabited corals than we would under the ecological constraints scenario as fish would have vacated low quality corals in favour of high quality corals.
This hypothesis looks at the idea that animals gain some benefit of remaining on a site that outweighs the benefits of dispersing. For example, the site might be of a high quality which improves the animal’s fitness to survive and reproduce. Dispersing from this site risks, losing this benefit, unless it can find a site which confers the same or better benefits. In my project, it is likely that there was a lot of variation in coral quality following the cyclone. While fish might have quickly moved into whatever shelter they could find, they might have realised later on that their coral was not very good (indicated by the green, algae covered coral in the diagram), and decided that it was more beneficial to vacate their low quality coral and move into a high quality coral (white coral in the diagram) with an existing group of fish. In this scenario, we would expect to find a lower proportion of inhabited corals than we would under the ecological constraints scenario as fish would have vacated low quality corals in favour of high quality corals.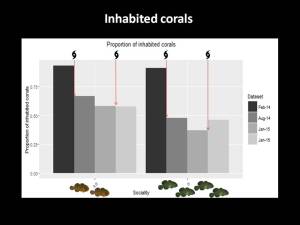 What we found was that after the cyclone, there was indeed a substantial drop in the proportion of inhabited corals. While this doesn’t definitively prove that benefits of philopatry are causing the observed social patterns, it does lend some support to the idea. There was also a drop in the proportion of inhabited corals for the asocial species, but it was not as substantial as that observed for social species. This likely due to a methodological ‘artefact’ which I won’t get into, but suffice to say, for social species, there is some support for benefits of philopatry playing a role in the observed social pattern following the first cyclone. Stay tuned for a more in-depth analysis of this data.
What we found was that after the cyclone, there was indeed a substantial drop in the proportion of inhabited corals. While this doesn’t definitively prove that benefits of philopatry are causing the observed social patterns, it does lend some support to the idea. There was also a drop in the proportion of inhabited corals for the asocial species, but it was not as substantial as that observed for social species. This likely due to a methodological ‘artefact’ which I won’t get into, but suffice to say, for social species, there is some support for benefits of philopatry playing a role in the observed social pattern following the first cyclone. Stay tuned for a more in-depth analysis of this data.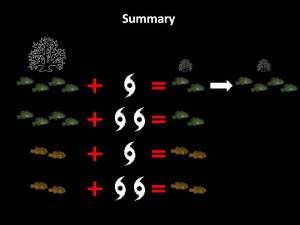 So, in summary, the major findings of this study were that after a cyclone, social species reduced in group size but asocial species did not. A year later social species had returned to their pre-cyclone group sizes, but in smaller corals. There is some evidence that benefits of philopatry are contributing to this pattern. The fact that asocial species did not alter their social organisation could indicate that the asocial strategy is either more robust to such an impact or that it is less flexible. Unfortunately, my surveys were not designed to examine patterns in abundance and I can’t really say whether either strategy is better or worse for recovery following a cyclone. This would be an interesting avenue for further research. Following a second cyclone, social species again decreased in group size, but did not return to pre-cyclone levels another year down the track. This might be because multiple impacts have longer lasting effects on social structure or because corals had reduced to such a small size that they were not capable of supporting larger groups.
So, in summary, the major findings of this study were that after a cyclone, social species reduced in group size but asocial species did not. A year later social species had returned to their pre-cyclone group sizes, but in smaller corals. There is some evidence that benefits of philopatry are contributing to this pattern. The fact that asocial species did not alter their social organisation could indicate that the asocial strategy is either more robust to such an impact or that it is less flexible. Unfortunately, my surveys were not designed to examine patterns in abundance and I can’t really say whether either strategy is better or worse for recovery following a cyclone. This would be an interesting avenue for further research. Following a second cyclone, social species again decreased in group size, but did not return to pre-cyclone levels another year down the track. This might be because multiple impacts have longer lasting effects on social structure or because corals had reduced to such a small size that they were not capable of supporting larger groups. Deep diving is something that I am passionate about – especially if it involves a shipwreck. It is a chance for me to push my skills, training and equipment and to explore some of the lesser dived or known sites. However this comes at a considerably higher risk which involves specialised training and equipment to manage safely. One of these risks is Nitrogen Narcosis – aka “the rapture of the deep”. Not a lot is known about the causes of nitrogen narcosis, but it is a narcotic effect brought on by the increased partial pressure of nitrogen in the breathing gas at depth. The threshold for nitrogen narcosis is different for each diver and can even vary dive-to-dive within a diver, depending on many factors. I usually enjoy getting a little “narc’d”. It can be quite euphoric and enjoyable if managed well. Nitrogen narcosis itself is not dangerous, unlike decompression illness, and there are no long lasting effects – no nasty hangovers. What can be dangerous though is the impaired actions of the diver experiencing euphoria. It’s essentially diving drunk. Nitrogen narcosis, like other narcotics can also present as paranoia and confusion which can lead to panic. Bad news in any depth of water. Thinking about these effects, I thought I’d share a story I wrote about a dive many years ago where I was overcome by narcosis paranoia in the hope that others can learn from my bad experience. This dive was a huge learning experience for me and still serves as a source of healthy anxiety before a deep dive. Nitrogen narcosis is not something that can be (nor should be) avoided, but it is certainly something to be aware of.
Deep diving is something that I am passionate about – especially if it involves a shipwreck. It is a chance for me to push my skills, training and equipment and to explore some of the lesser dived or known sites. However this comes at a considerably higher risk which involves specialised training and equipment to manage safely. One of these risks is Nitrogen Narcosis – aka “the rapture of the deep”. Not a lot is known about the causes of nitrogen narcosis, but it is a narcotic effect brought on by the increased partial pressure of nitrogen in the breathing gas at depth. The threshold for nitrogen narcosis is different for each diver and can even vary dive-to-dive within a diver, depending on many factors. I usually enjoy getting a little “narc’d”. It can be quite euphoric and enjoyable if managed well. Nitrogen narcosis itself is not dangerous, unlike decompression illness, and there are no long lasting effects – no nasty hangovers. What can be dangerous though is the impaired actions of the diver experiencing euphoria. It’s essentially diving drunk. Nitrogen narcosis, like other narcotics can also present as paranoia and confusion which can lead to panic. Bad news in any depth of water. Thinking about these effects, I thought I’d share a story I wrote about a dive many years ago where I was overcome by narcosis paranoia in the hope that others can learn from my bad experience. This dive was a huge learning experience for me and still serves as a source of healthy anxiety before a deep dive. Nitrogen narcosis is not something that can be (nor should be) avoided, but it is certainly something to be aware of.


















